9 Tricks You Can Use to Improve Your Sort
Sorting is a complicated art, not just from the point of view of the technology and setup involved, but also in the way that you prepare your cells. What you do to your cells prior to bringing them to the sorter will play a very important role in the success of the sort.
In some ways it is analogous to painting - if you slap some paint over the top of the crumbly old paint in your house, it'll work, but the outcome will be less than satisfactory. However, if you take the time to sand back the old surface, cut in the edges, clean and prepare the surface, you will end up with a paint job that serves you well for a long time. And this theory applies to your sorting. By the time you bring your cells to the sorter, 90% of the hard work should be over.
So what can you do to prepare your cells in the best way? Most of these tips require so little effort you won't even notice that you are doing them, but they will make a world of difference.
1. Use Polypropylene Tubes
A large number of your cells will be lost simply by sticking to the wall of the sort tube - you can reduce this by using polypropylene tubes instead of polystyrene tubes.
Likewise, ensure that your collection tubes are polypropylene.
If you use polystyrene tubes, during the sort the charge applied to the deflected droplets will build up around the polystyrene tubes, and can result in a charged field that causes cells to "bounce out" of the collection tube.
2. Sort Sub-Confluent Cell Lines
You want to sort as many cells as you can, so you grow your flask up until cells are practically bursting out of the seams. Great, millions of cells into the sorter means millions of cells out! Sadly, this is rarely the case. If you can keep your cells growing in log-phase before their confluence inhibits their divisions, your cells will not only be happier and more robust, but the sort outcome will be significantly better.
This is particularly important with adherent cell lines. Once they are fully confluent some of the cells will enter senescence, and are not likely to survive the rigours of the sort process. If you desperately want millions of cells, keep your cells growing to sub-confluence in the passages leading up to the sort, and if necessary grow up a couple of flasks at a lower density than one at maximum density.
3. Know your cells
Image from Blausen.com staff (2014). "Medical gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. (Own work) [CC BY 3.0], via Wikimedia Commons
Different cell types will require different pre-sorting preparation by you, different sorting buffers to optimise cell happiness and may also require special handling at the sorter.
Speak with your core staff about your cells. Even if they haven't sorted your exact cell line/type before, they will have had experience with similar cells and will have recommendations based on their operating procedures and your sorting requirements. This also helps to ensure that the staff have the required information for setting up the sorter in the best way for your cells.
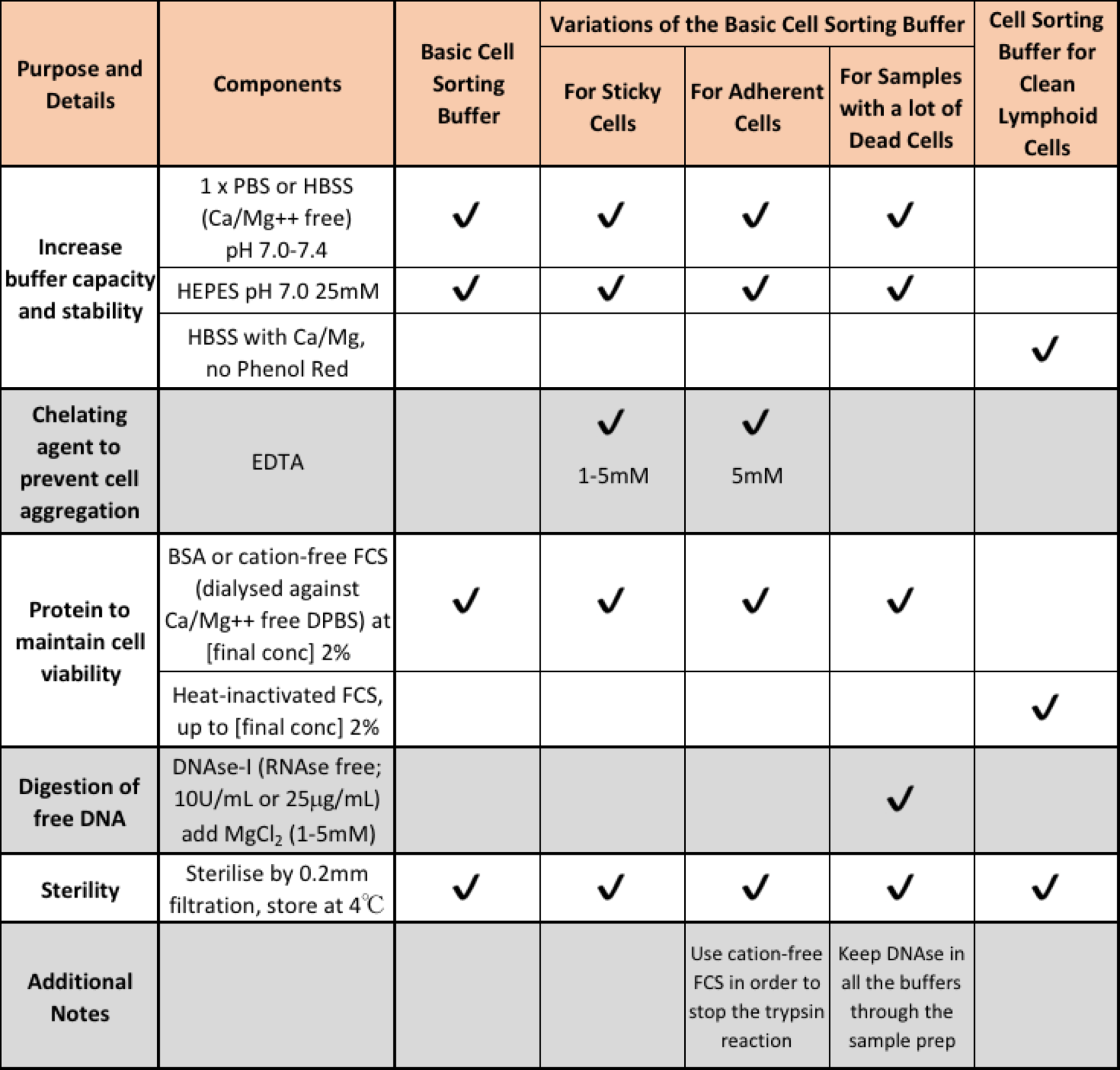
Use an appropriate sorting buffer
Let's call this tip 3.5.
Using incomplete culture media, while often the simplest choice for sorting, is not usually a good choice, nor is using plain PBS. Cell sorting is stressful so the cells are going to need to have access to some protein in the sort buffer to prevent them from apoptosing in response to a nutrient-depleted environment, therefore maintain at least 2-3% FCS in your sort sample tube to keep your cells content.
Why not more protein? The reason behind this is 2-fold: if your media contains plenty of protein then adherent cells will have all the resources they require to adhere together again, undoing all the hard work you did trying to get them into a single cell suspension. Secondly, significant protein content in the buffer can affect the optical properties of the sample as well as disrupting the stream stability. Stream disruption through blockages or premature break off will affect your sample purity and recovery.
Another important ingredient in sorting buffers is HEPES. Culture media like RPMI is formulated to effectively buffer in the atmophere of your incubator (e.g. in high CO 2 atmosphere). HEPES buffer is designed to work in a normal atmosphere, likewise, HBSS is optimised for buffering in a normal atmosphere.
The table below outlines a collection of recipes designed to help you pick the best recipe for your sorting experiment.
Adapted from Buffer Suggestions for Cell Sorting from FCRC at Rockefeller University
4. Know the frequency of the fraction you wish to recover
- You want 1 million cells for a downstream application.
- You present your trusty core staff with a tube of 10 million cells.
- Your positive fraction is 1% --> 1% of 10 million is 100,000.
- Best case scenario here: assume that you can recover about 50,000 from the sorter (loss is due to electronic aborts, doublet aborts and cell death during and after sorting). At this point you are going to be disappointed with your sort outcome.
If you have the opportunity to run your cells through an analyser to identify and quantify the fraction of interest prior to the sort, you will know how many cells you need to start with to collect the desired number of cells.
5. Filter AND Count your cells... and resuspend them at the recommended concentration
The most important factor here is to count AFTER you have prepared and filtered your cells.
Filter your sample, preferably through a 30µm filter and resuspend your cells at the concentration recommended based on your cell type. If you don't filter your cells, you may find yourself on the receiving end of some dirty looks from your sort operator who will be unimpressed by the blocking hazard that your cells represent.
The optimal sort speed is dictated by the frequency of the droplets created by the sorter and sorter setup is dictated by your cell type. If you resuspend your cells at the recommended concentration the sort will be optimised for both efficiency and speed.
Speak with your local core staff about their preferences, but as an example, at the DFCF we recommend the following:
- If sorting cells that will be processed with the 70µm nozzle, resuspend cells at 4 x 10 7 /mL
- If sorting cells that will be processed with the 85µm nozzle, resuspend cells at 2 x 10 7 /mL
- If sorting cells that will be processed with the 100µm nozzle, resuspend cells at 1 x 10 7 /mL
If you don't have that many cells in total, the minimum volume required in your sorting tube is 200µL.
Always bring some spare sorting media, then if cells prove troublesome they can be diluted to a more cooperative concentration.
6. Always Use a viability dye
In this example the post-sort purity check showed a GFP+ fraction that appeared to be lower than the pre-sort expression. By adding a viability dye we were able to determine that the GFP- fraction were cells that had died during the sort process, and in fact the post-sort purity of viable cells was 99.4%.
I can't think of a single instance where an experiment was harmed by the addition of a viability dye, but I can think of many where an experiment was rescued by the addition of a viability dye.
Every experiment should contain a viability dye.
Viability dyes come in so many flavours now, you should always be able to slot one into your panel, even your multicolour panels. Chat to your local core staff about recommendations that will work with your experiment and their instruments. This tip applies to analysis work as well as sorting.
7. Always provide the requested/required controls
Every experiment, even the boring GFP-only ones require controls. Controls are essential to ensuring that what you think you are sorting and what you actually receive are the same thing. Helpful controls include negative controls, positive controls, unstained cells, untransfected cells, compensation controls, FMOs and treatment controls, just to name a few.
If you are unsure about what controls should be used in your experiment, talk to your core staff about your plans and they can help you out.
8. Be clear about your downstream requirements for the cells
At the cell sorter we can make a number of decisions that will impact the number and potential purity of the cells that will land in your tube.
The major player here is the instrument Threshold.
If you plan to culture your cells the threshold can be raised. This will mean that dead cells and debris fragments will be ignored by the sorter and as such may be sorted into your tube alongside the cells of interest. By "ignoring" the debris the sort efficiency is improved and you are likely to recover more of your preferred cells of interest. This results in a reduction of the purity of the total events in the tube, however, the purity of the "viable" cells is still as expected. Unless you have mutant zombie cells, the dead cells will not grow up in culture while your cells of interest will expand, so there should be little impact downstream by including this debris in the collection tube.
On the other hand, if you intend to use your cells for a functional assay or perform nucleic acid analysis or protein expression studies, during the sort we need to make every effort to ensure that debris and dead cells are not included or they can skew your results. In these cases the threshold is dropped to ensure that the debris and dead cells are properly assessed by the instrument. The downside to this is that your sort efficiency is likely to drop as well, reducing your overall recovery.
9. Don't leave your cells in the collection buffer!
Sheath fluid is excellent for clean operation of the cell sorter but not so much for long-term cell survival!
When preparing collection tubes for catching your cells, ensure that you provide your core staff with tubes that are HALF FULL (yes, half full!) of collection media that your cells like to grow in and supplement the media by doubling the amount of FCS that you would normally use. The higher protein content of the collection buffer helps keep cells happy prior to their return to your lab.
If collection tubes are empty or have insufficient media in them, the chance that a cell will strike the wall of the tube rather than landing in the media is significantly increased. Cells that don't land in the media are unlikely to survive.
When you get back to your lab, spin your cells down as soon as you can and then put them into fresh culture media, sometimes with a little boost in protein % if they are particularly fussy. They will thank you for the extra pampering. Well, perhaps they won't enjoy the centrifugation part, but the rest of the TLC will be appreciated.


![Image from Blausen.com staff (2014). "Medical gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. (Own work) [CC BY 3.0], via Wikimedia Commons](https://images.squarespace-cdn.com/content/v1/59d0bff1cd39c3d497ea3ca1/1507515936994-WW3QSSTHYYMKK34DQ8NL/Blausen_0909_WhiteBloodCells+%281%29.png)